The Chemistry of Chocolate
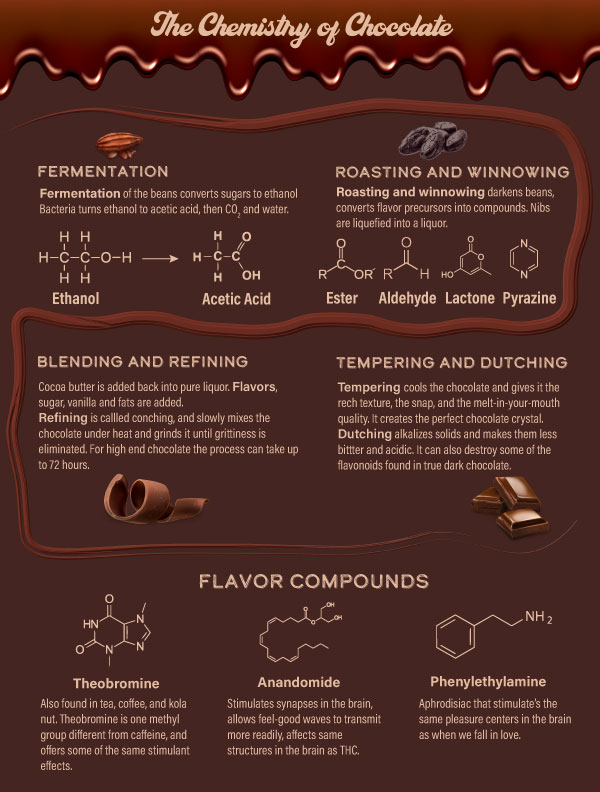
Chocolate is one of human kind's favorite indulgences. But did you know that the cocoa beans have to go through several chemical transformations to become the chocolate that you know and love? Without these processes, the seeds would be bitter, rough and bland. The brain interprets the natural bitterness of chocolate as a poison, making it unpleasant to eat without processing and adding sugar.
The nibs of the cocoa seeds are what we use to make chocolate. Fermentation must begin within 48 hours of the pod being opened, and takes 5-7 days. Microorganisms then form and remove pulp from the seeds. From there, the complicated process of extracting the deliciousness from these nibs begins.
With fermentation comes enzyme activity, oxidation and the breakdown of proteins into amino acids. This is where the flavor precursors begin to form.
Next, beans are cleaned and roasted, removing the vinegar smell that fermentation causes. Winnowing machines remove bean shells and leave just the nib. Nibs are ground and liquefied into cocoa liquor.
Blending and refining takes place after roasting, then tempering or cooling. Tempering gives the nice texture that snaps and then melts in your mouth.
Dutching alkalizes cocoa solids to make them less bitter and acidic for cooking or powdered chocolate.
After this complicated process, we end up with that irreplaceable flavor we all know and love.
We hope this infographic gives you even more appreciation for the brilliant chocolatiers, or perhaps, chocolate chemists, who bring this delightful treat into our lives.